Surgical tools, as well as machinery, aid medical treatments as well as, are vital to the diagnosis as well as therapy of individuals. In the majority of medical facilities and clinical centers, a qualified biomedical as well as design team takes care of the whole clinical stock and is accountable for taking care of clinical tools hazards. Here is the stock that they handle:
- Analysis devices: This is utilized to examine a client’s medical condition as well as consists of X-ray as well as MRI equipment.
- Healing equipment: This is utilized to deal with as well as minimize injuries. It consists of physical rehabilitation as well as ultrasound, electrotherapy devices, as well as laser therapy makers.
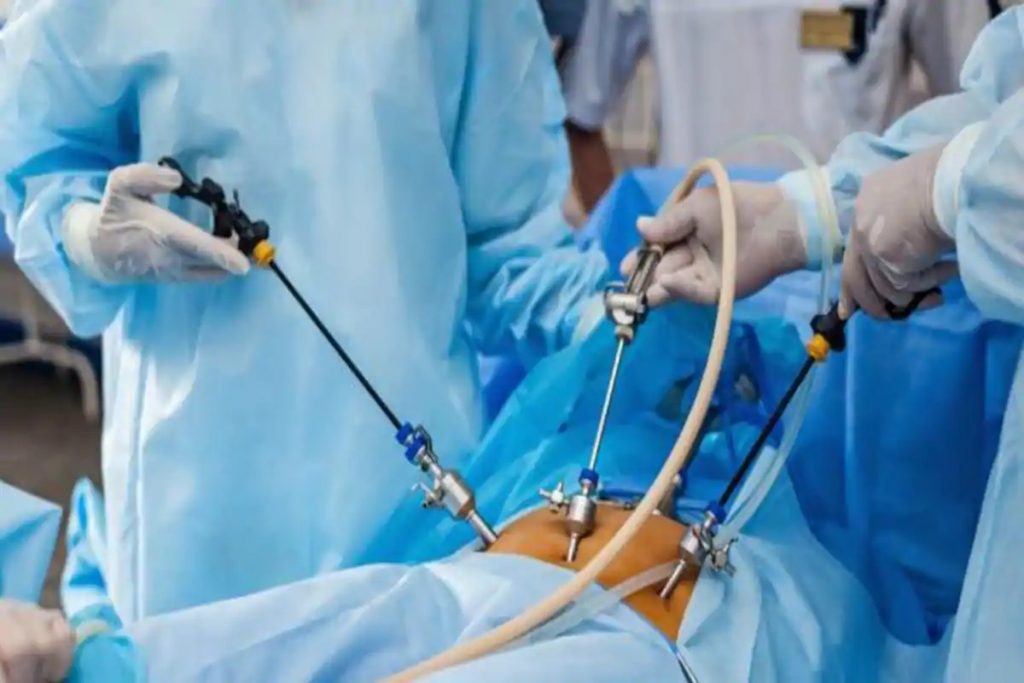
- Surgical instruments: These are smaller sized devices that can be separated into the following types:
-
- Cutting as well as holding tools
- Retractors
- Homeostatic forceps
- Clamps as well as distractors
- Implant accessories
Being in charge of such a comprehensive array of tools can be troublesome, specifically when the risks are so high. Not only does careless monitoring as well as administration lead to inadequacy, but it can seriously have personal results. As an example, inadequate upkeep enhances the possibilities of downtime, as well as poor servicing and also sanitation, which can be hazardous to both doctors as well as people.
This is why it is vital to establish some standard Karl Storz medical instruments’ security as well as service guidelines. Below, we’ll take you with some typical issues around medical devices, as well as layout ideal practices on how you can make sure efficiency whatsoever times:
Function in the direction of making your facilities secure for all
An area that is implied to offer like clients ought to not be a source of risk to their health and wellbeing. This is why healthcare facilities need to proactively guarantee that their centers are compliant with health and wellness laws, as well as do not present a danger to people as well as employees alike.
You can decrease medical equipment risks by complying with these three actions:
- Pre-market control where you carry out professional tests for all devices.
- Make sure that your vendor is registered to reduce the opportunities for acquiring subpar equipment.
- Dispose of equipment as well as instruments when it’s time for them to go.